1 Amu To Kg
1.9926468 x 10¯ 26 kg/atom / 12 amu/atom = 1.660539 x 10¯ 27 kg/amu (this is the mass of 1 amu) 2) Use Einstein's mass-energy equation to determine Joules in one amu: E = mc 2.
Convert 1 amu to MeV
- 1 amu is equal to 1.6603145E-27 kilogram. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between atomic mass units and kilograms.
- Kilogram (abbreviations: kg, or kgm): is a SI (metric) system unit of mass (IPK, also known as 'Le Grand K' or 'Big K') and equivalent to 1/1000 of one gram.Where, at the temperature of melting ice, one gram is the absolute weight of a volume equal to the hundredth part of a metre cube of pure water.
- The SI unit of mass is a kilogram, which is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10⁻³⁴ when expressed in the unit J s, which is equal to kg m² s⁻¹, where the meter and the second are defined in terms of c and Δν Cs.Multiples of kilogram are also commonly used, such as a gram (1/1000 of a kilogram) and a tonne.
- Why create a profile on Shaalaa.com? Inform you about time table of exam. Inform you about new question papers. New video tutorials information.
Solution:

1) Determine mass of 1 amu (in kg):
By definition, the mass of 1 atom of C-12 is 12 amu.Therefore, the mass of one mole of C-12 atoms is, by definition, 12 g
Avogadro's Number is 6.0221409 x 1023 mol¯1

12 g/mol / 6.0221409 x 1023 mol¯1 = 1.9926468 x 10¯23 g (mass of one atom of C-12 in g)
(1.9926468 x 10¯23 g) (1 kg / 1000 g) = 1.9926468 x 10¯26 kg (mass of one atom of C-12 in kg)
1.9926468 x 10¯26 kg/atom / 12 amu/atom = 1.660539 x 10¯27 kg/amu (this is the mass of 1 amu)
2) Use Einstein's mass-energy equation to determine Joules in one amu:
E = mc2E = (1.660539 x 10¯27 kg) (2.99792458 x 108 m/s)2
E = 1.49242 x 10¯10 kg-m2/s2 (this is Joules)
3) Convert J to eV, then MeV
1 eV = 1.60217733 x 10¯19 J1.49242 x 10¯10 J / 1.60217733 x 10¯19 J/eV = 931494893 eV
What Is An Amu
(931494893 eV) (1 MeV / 106 eV) = 931.494893 MeV
Often, 931.5 MeV is the value used in problem solving.
4) 931.5 MeV is the energy in one amu. Is it possible to express the mass of one amu using MeV in some way? I'm glad you asked:
Atoms To Amu Calculator
E = mc2931.5 MeV = mc2
m = 931.5 MeV/c2
The use of 931.5 MeV/c2 for the mass of 1 u is wide-spread. So much so that the c2 is often not written and you must infer its presence by context.
5) To sum up:
1 u = 931.5 MeV (expressed as energy)
1 u = 931.5 MeV/c2 (expressed as mass)
In solving problems, you may see this unit:
931.5 MeV/u-c2read it as 931.5 MeV/c2 per 1 u
By the way, if you look around the Internet, you will find values different than the 931.494893 value I calculated. This is because different authors will use values that they have rounded off differently than the ones I used.
For example, I used 1.660539 x 10¯27 kg for the mass of 1 amu. Often, you will see it as 1.66 x 10¯27 kg.
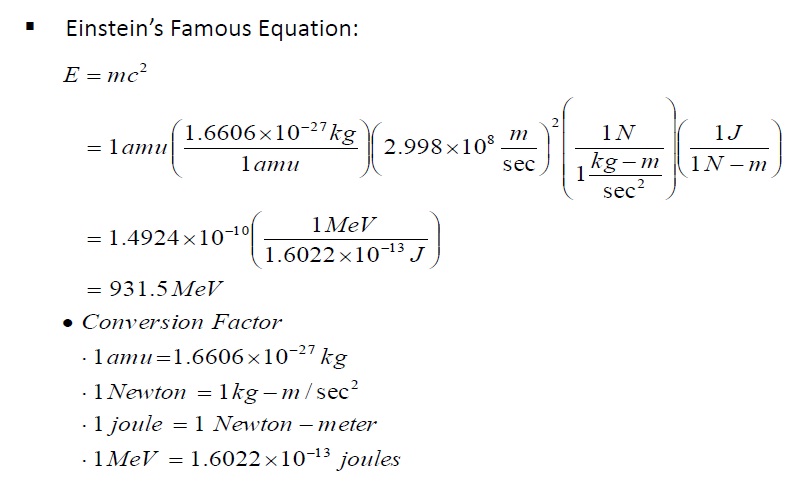
The cumulative effect of these rounding off decisions will be seen in slightly different answers from different authors.
