Atom Is Electrically Neutral
Why Atom Is Electrically Neutral
June 2010 Chemistry Regents #54-55
An atom is electrically neutral when it has the same amount of electrons as protons. Atom= one part of an element in a molecule- there are two atoms of hydrogen and one atom of oxygen in h20- water. Neutron= a neutrally-charged particle in the nucleus of an atom. Electron= a negatively-charged particle that flies around the nucleus of an atom. The atom is electrically neutral, because it has an equal number of protons and electrons, but the electrons are all on one side of the atom, so a dipole (uneven distribution of charge) is created. It is temporary, because the electrons continue to move. Based on your answer to #30, what do you think helps neutral atoms and molecules.
Highlight to reveal answers and explanations
An atom is electrically neutral because the overall charge of an atom is zero. The atoms are made of three subatomic particles called protons, electrons and neutrons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.
Questions | ||
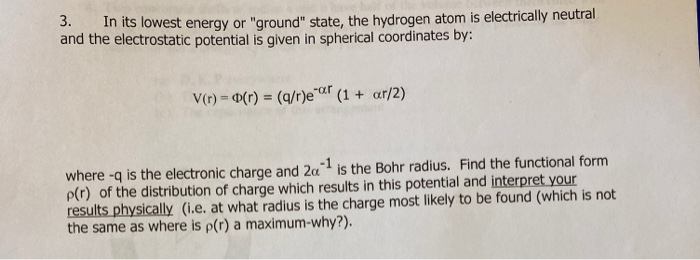
Base your answers to questions 54 and 55 on the information below.
|
| Back to Regents Exams |
Click to see full answer.
Also, why is an atom electrically neutral quizlet?
An atom is electrically neutral because the number of negatively charged electrons outside the nucleus equals the number of positively charged protons inside the nucleus. You can only create an ion by adding or removing electrons from a neutral atom.
Subsequently, question is, is the nucleus of an atom electrically neutral? An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. The nucleus of an atom contains protons and neutrons.
Likewise, people ask, what elements are neutral atoms?

A neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. Example: Carbon has 6 protons. The neutral Carbon atom has 6 electrons.
Why neutron is electrically neutral?

Explain Why Atom Is Electrically Neutral
Neutrons are the particles in an atom that have a neutral charge. They aren't positive like protons. They aren't negative like electrons. So, if an atom has equal numbers of electrons and protons, the charges cancel each other out and the atom has a neutral charge.